Our
Publications
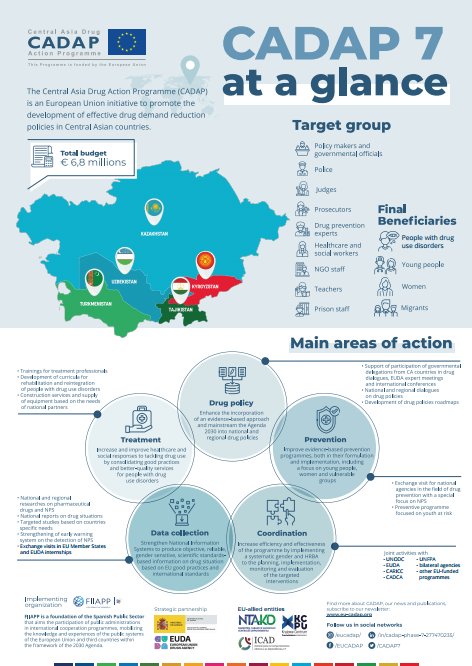
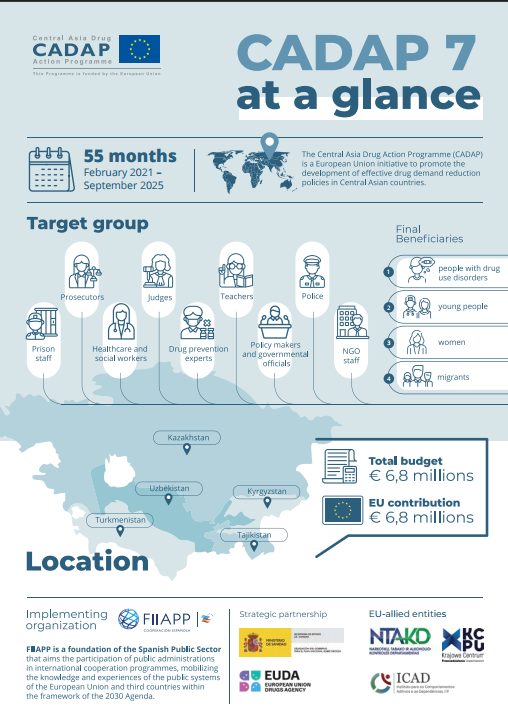
CADAP

Overview of early warning systems in countries of Central Asia
In cooperation with the CADAP countries, Společnost Podané ruce has played a critical role in providing technical assistance for the enhancement of data collection and...